
|
Biological Magnetic Resonance Data BankA Repository for Data from NMR Spectroscopy on Proteins, Peptides, Nucleic Acids, and other Biomolecules |
Member of
|
NMR spectroscopy data on natural compounds from the DTP Repository at The National Cancer Institute are available at BMRB Metabolomics database
Developmental Therapeutic Program (DTP) of NIH National Cancer Institute (NCI) maintains a Repository of synthetic compounds and pure natural products that are available to investigators for research purposes. The Repository collection is uniquely diverse and contains the compounds that have been either submitted to DTP for biological evaluation or, in some cases, synthesized under DTP auspices. For years the NCI has acquired the majority of its compounds through the contributions of chemical and pharmaceutical industries, research organizations and government laboratories. Both domestic and foreign sources have contributed. The NCI Natural Products Open Repository Program enables the scientific community to investigate the materials as potential sources of agents for the treatment of all human diseases. The availability of numerous compounds resulted in re-invigoration of natural product based research. The collection covers the plant, marine invertebrate, microbial derived samples and represents one of the largest sources of a diversity of natural compounds available for screening.
The multicultural body of medicinal texts on traditional methods of treatments represents a rich source of empirically tested therapeutics based on natural compounds. It is an excellent source of inspiration for discovery of novel bioactive molecules. In modern research, individual compounds are isolated from medicinal herbs and investigated as a potential new lead for drug discovery. An example that highlights the great potential gains from this strategy is the recent discovery and application of bioactive alkaloid camptothecin.
Camptothecin is isolated from the stem wood of the Camptotheca acuminata that has been used in traditional Chinese medicine formulations for thousands of years. This compound selectively inhibits the nuclear enzyme DNA topoisomerase, the human enzyme that is being investigated as a target for cancer chemotherapy. Several semi-synthetic analogs of camptothecin have also demonstrated similar or enhanced anti tumor activity. The FDA has approved the camptothecin derivatives topotecan and irinotecan for second line treatment of some types of cancer. These and other derivatives have become part of the medical research dedicated to finding better chemotherapeutic agents with lesser tissue toxicity [1].
There are multiple examples of naturally derived bioactive compounds, applied in cancer research, such as secondary plant metabolite parthenolide and secondary metabolite stictic acid found in some lichens. Parthenolide, a naturally occurring sesquiterpene lactone derived from feverfew (Tanacetum parthenium), exhibits exceptional anti-cancer and anti-inflammatory properties, making it a prominent candidate for further studies and drug development. Acting as a covalently reactive compound, it displays anti-inflammatory, redox-modulating, and epigenetic activities, as well as selective cytotoxicity towards cancer stem and progenitor cells. It has been reported that parthenolide shows strong suppression of pro-apoptotic genes. This compound acts both at the transcriptional level and by direct inhibition of associated kinases. Similarly, the parthenolide-induced reactive oxygen species-mediated apoptosis of tumor cells via the intrinsic apoptotic signaling pathway, are observed. The unique ability of this compound to not harm normal cells but at the same time induce sensitization to extrinsic as well as intrinsic apoptosis signaling in cancer cells provides an important, novel therapeutic strategy for treatment of cancer and inflammation-related disorders [2].
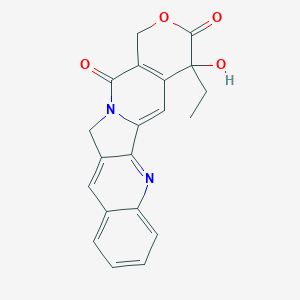
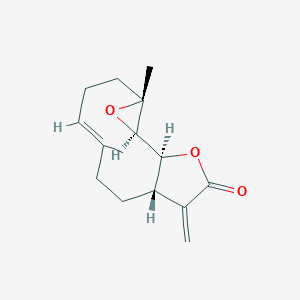
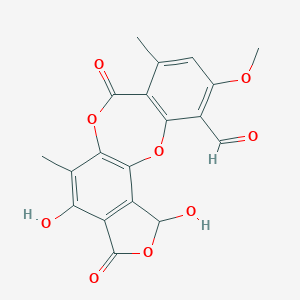
Fig 1.Molecular structures of camptothecin, parthenolide and stictic acid.
Another anti-cancer drug candidate, stictic acid, is an aromatic organic compound of depsidone molecular structure, a product of secondary metabolism in some species of lichens (Fig 2). Stictic acid is the subject of preliminary biomedical research that showed cytotoxic and apoptotic effects in vitro. Stictic acid also showed neuroprotection through the antioxidant activity by decreasing the peroxide moieties concentration in lipids oxidation process. Growth inhibition of several cancer cell lines was tested in models of potential anticancer activity of stictic acid. Results demonstrated the strong potential of this compound as an anticancer agent [3].
Fig 2.The lichen species Sticta hypochroa Vainio, the source of stictic acid. Photographed in Parque Nacional Queulat, Aisen, Chile. Source: http://commons.wikimedia.org/w/index.php?curid=6410705
Natural products such as described above, have contributed immensely to new drug development in the recent times and continue to do so. Nevertheless, complications arise due to the fact that the amounts of bioactive components in plants are low. The elaborated and often state-of-art isolation and purification techniques have been the limiting factors in implementation of natural products in drug development. There are constant urgent needs to develop effective and selective analytical methods. As the methods continue to develop, more and more new automatic and rapid techniques have been created to extract and separate natural products, that might reach the requirement of high-throughput screening.
As a standard procedure, the NMR spectroscopy based analytic techniques are applied in the processes of the compounds identification, purity assessment and quality control. Furthermore, NMR spectroscopy is increasingly used as a technique to provide insight into mixtures of natural products belonging to the same or different chemical classes, without prior separation of the individual components. The sample preparation for NMR is simple and nondestructive. In this context NMR methods are being widely used with success in the structural identification of such natural products as camptothecin, parthenolide and stictic acid as well as various mixes of compounds.
The metabolites NMR spectra database at the BMRB contains 1D and 2D NMR spectroscopy data for secondary metabolites camptothecin (bmse001324), parthenolide (bmse001294), stictic acid (bmse001310), and for a a number of other compounds provided by the Repository of synthetic compounds and pure natural products at Developmental Therapeutic Program (DTP) of NIH National Cancer Institute (NCI). The time-domain data archives, the spectra pictures as well as the tables of peak transitions and assigned chemical shifts are available. Beside direct applications in biomedical research, the data are of interest for building of the modular suites of exercises for laboratory sessions in physical chemistry and instrumental analysis, and specifically useful for teaching the 1D and 2D 1H-13C NMR data assignments in methods of isolation, purification and characterization of natural compounds, by providing complete sets of reference data as well as visual context.
[1]
Chemovirotherapeutic Treatment Using Camptothecin Enhances Oncolytic Measles Virus-Mediated
Killing of Breast Cancer Cells. Sci Rep 9, 6767 (2019) ; Chen-Jei Tai et al
doi:10.1038/s41598-019-43047-3
[2]
Parthenolide, a Sesquiterpene Lactone, Expresses Multiple Anti-cancer and Anti-inflammatory
Activities; Mathema, V.B., Koh, Y., Thakuri, B.C. et al. Inflammation 35, 560–565 (2012)
doi:10.1007/s10753-011-9346-0
[3]
Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens;
Correché ER et al. Altern Lab Anim. 2004 Dec; 32(6):605-15.
doi:10.1177/026119290403200611
